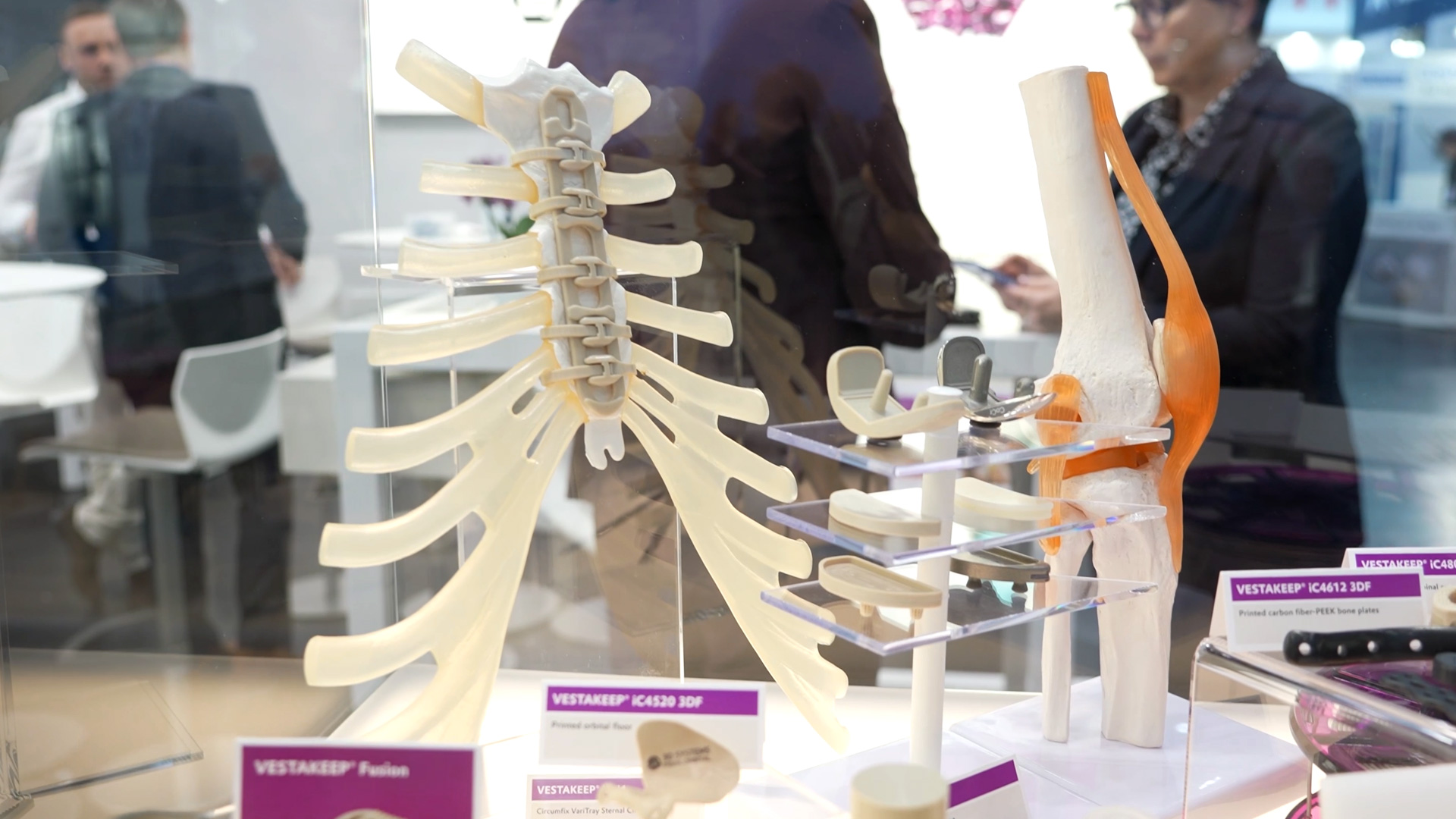
Trelleborg presents integrated solutions for high-risk cardiac implants
by A. Bergmeier - 2025-12-01The exhibition at Compamed illustrates how crucially important precision medical technology has become for patients with severe heart disease. This year, the company is presenting a product display that showcases its entire vertical range of manufacture for cardiac applications – from individually manufactured microcomponents to fully packaged implants.

Integrated cardiological components and implantable systems
The display brings together a range of products that clearly illustrates the technical complexity of modern cardiac medicine. It ranges from long-term implantable components and micro-precise tubing systems to elements that are ultimately required in packaging technology for sensitive products. The key concept is that of an integrated solution: All work steps—from raw material to labeled end product—can be mapped within a controlled process chain.

Product diversity for pacemakers, pumps, and microimplants
One focus is on technology for pacemakers and mechanical pump systems. This includes spiral-shaped tubes whose internal structure enables the insulation of the finest wires. These designs protect the cables of the so-called leads that lead into the pacemaker. They are supplemented by micro-manufactured anchors that securely fix these wires in the heart tissue. Connectors for heart pumps are also among the solutions on display. These components are sutured directly to the heart and ensure a permanent connection to pump systems that support the pumping performance.
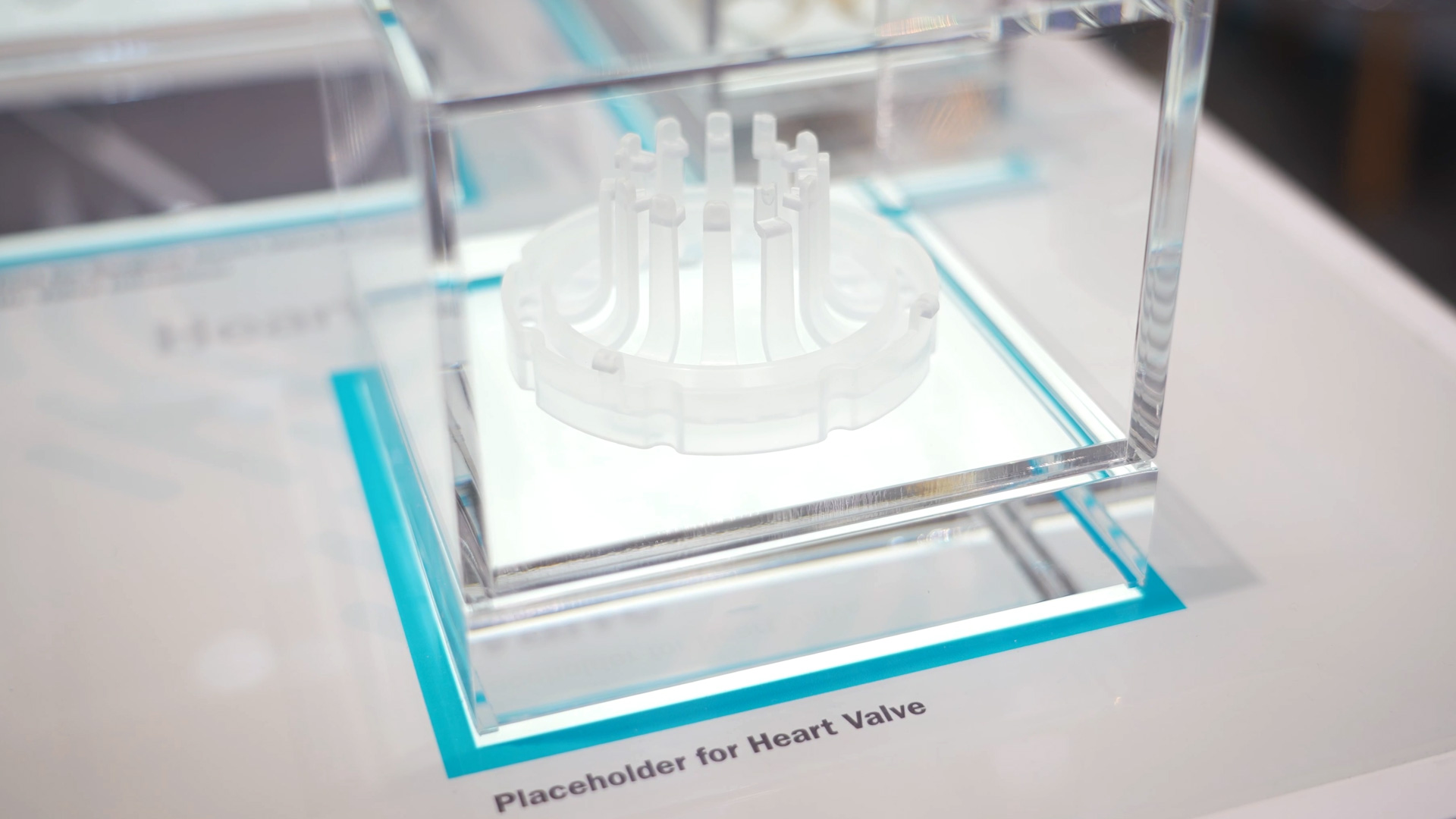
Quality control for high-risk components in cardiology
The smaller the systems become, the greater the requirements. Implantable devices should be inconspicuous, portable, and as unnoticeable as possible—at the same time, they must function reliably over the long term. This is precisely where the importance of consistent quality processes becomes apparent. Every critical component undergoes a complete inspection. Testing begins during the manufacturing process: measuring systems detect deviations in the micrometer range, material tests ensure durability, and validations check the performance of the entire production step.

Finally, a manual, visually supported inspection is carried out by specialist personnel. Some characteristics can only be reliably assessed through experience, which is why this step is an important part of the overall system. Depending on the complexity of a component, additional validation stages are added to ensure that no defective components reach customers or patients.

Miniaturized heart implants and micromechanical precision
Many developments in recent years show a clear trend: implants must become smaller. Today's pacemakers have compact housings and extremely fine components that must still function accurately and reliably for many years. Pump systems that used to rely on bulky external devices can now be operated with small, portable battery packs. This miniaturization requires designs that are technically sophisticated and yet remain robust. The focus is therefore not only on functionality, but also on suitability for everyday use. Patients should be able to lead as normal a life as possible despite cardiac limitations – with less stress, more freedom of movement, and a higher quality of life.
Manufacturing technologies for insulated leads and spiral tubing systems
Complex tubing geometries with multiple lumens that are twisted within themselves are among the technological examples that can only be developed through decades of expertise. Several fine channels are brought together in a micromechanically sophisticated structure. Such components are essential for guiding sensitive lines while withstanding movement and stress in the human body over the long term.

Validated manufacturing processes for pump connectors and critical components
Components that are in direct contact with heart tissue require a high degree of process stability. Connectors for pumps are one such component. In addition to material selection and manufacturing precision, validation plays a central role here. Each process stage is documented, tested, and only approved once performance is reproducible. This approach ensures that every component achieves the same functional reliability.
Trelleborg expertise for high-risk cardiological applications
The presentation showcases technologies that have taken decades to develop. This includes not only material expertise, but also experience in markets with the highest requirements. The international setup enables customer-specific solutions to be implemented locally while adhering to globally coordinated quality standards. For manufacturers, this means reliable availability and clearly calculable development processes.